Why Would Use Ph 5.6 to Describe Acid Rain
It is not considered that harmful. Rainwater with a pH less than 56 is called Acid rain.
Sulphur Dioxide and Nitrogen Oxide are both gases which are emitted into the atmosphere due to the burning of fossil fuels mainly in industry and from road transport.

. This is because it is exposed to the carbon dioxide in the atmosphere. It is the rain fall and other forms of precipitation with a pH of less than 5 of normal rain is 56-65. 6 it is called acid rain and it is due to the presence of.
Good pH for calcareous lakes. When it rains rain water passes through the air which has small amount of carbon dioxide. 41 a i State one cause of acid rain other than that shown in Fig.
IUPACs definition Rain with pH values about 5. While you may assume that water is a neutral pH the dissolved carbon dioxide in the rain naturally creates a slightly acidic pH of approximately 56. Acid rain has a pH value of 30 whereas normal rain has a pH value of 56.
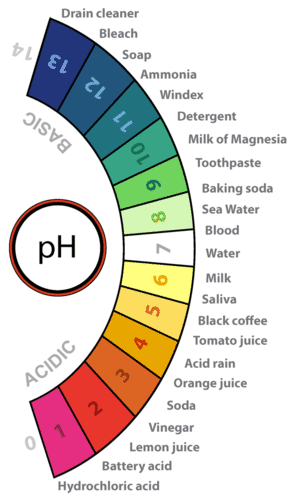
Power stations and factories release sulfur dioxide rain becomes acidic harming vegetation and organisms that live in water wind-blown chemicals combine with water vapour in the air lake Fig. When sulphur dioxide and nitrogen oxides are released into the air they react with water and oxygen and produces sulphuric and nitric acids. The lower a substances pH less than 7 the more acidic it is.
Typical acid rain has a pH value of 40. 6 because of the presence of H ions produced due to the dissolution of C O 2 in rain water from the atmosphere. Acid rain refers to the presence of excessive acid content in the atmosphere and also in the precipitation water rain snow mist fog etc.
Normal rain has a pH of about 56. Normal clean rain has a pH value of between 50 and 55 which is slightly acidic. Normally the pH of rain water is 5.
Acid rain can have pH values near 4. Difficult to reproduce breathe interference with Ca2 uptake. When the pH of the rain drops below 56 it becomes acidic.
The biggest sources are coal-burning power plants factories and automobiles. Normal rainwater has a pH of 56 slightly acidic. Be originating somewhere.
Actually the acidity of rain water is increased due to the large quantity of SO2 and oxides of nitrogen present in the air. When pH of rain water becomes less than 7 or equal to 56 the rain water becomes acidic. But acid rain can have pH levels lower than 43-where is the extra acidity coming from.
Commonly results from acids formed from pollutants. The presence of this acid makes rain water slightly acidic. Sulphur Dioxide comes from sulphur impurities in the fossil fuels.
PH or pure rain water is 7 neutral When the rain water comes in contact with the CO₂ gas present in the air it leads to the formation of Carbonic acid which is a weak acid. 500 g of an impure sample of hydrated ethanedioic acid COOH22H2O was dissolved in water to make 100 dm3 of solution. Acid rain effect on aquatic organisms.
When it dissolves in pure water rain water it makes it acidic. During a rain storm rain comes straight down with velocity Vi -15. Acidity is measured by pH with 70 as neutral lower than 70 acidic and higher than 70 alkaline.
Liquids with a pH less than 7 are acid and those with a pH greater than 7 are alkaline or basic. Rain is called as acid-rain when its pH is less The normal rains pH is 56. Normal clean rain has a pH value of between 50 and 55 which is slightly acidic.
Clean or unpolluted rain has a slightly acidic pH of 56 because carbon dioxide and water in the air react together to form carbonic acid a weak acid. Ability of a watershed to neutralize the acid. Thus pure rain in equilibrium with the atmosphere has about mathrmpH565.
You are forgetting an important component of the air. Acid rain usually has a pH. 1 ii Describe two effects of acid.
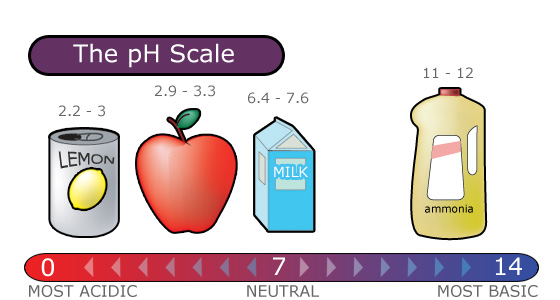
Normal rain is slightly acidic with a pH of 56 while acid rain generally has a pH between 42 and 44. The higher a substances pH greater than 7 the more alkaline it is. The carbon dioxide gets dissolved in the rainwater and forms carbonic.
Part of the country. 41 shows a cause of acid rain. Once nitric and sulfuric acids are added into the mix the acidity level of rain can drop to 42.
This is because it reacts with carbon dioxide in the atmosphere and forms mildly acidic carbonic acid before it turns into rain. It is mainly caused by anthropogenic activities. The more accurate term is acid precipitation Distilled water which contains no carbon dioxide has a neutral pH of 7.
The extra acidity must. However when rain combines with sulfur dioxide or nitrogen oxidesproduced from power plants and automobilesthe rain becomes much more acidic. CO 2 H 2 O H 2 CO 3.
In this heavily industrialized. 250 cm3 samples of this solution were titrated against a 0100 mol dm-3 solution of sodium hydroxide using a suitable indicator. The normal rain is slightly acidic having a pH of about 56 as carbon dioxide gas reacts with it to form a weak carbonic acid.
Rain -water normally has a pH of 56 due to the formation of carbon dioxide present in the atmosphere. However in many areas of the United States acid rain is now the norm because of pollution emissions from sources such as coal-fired power plants and car exhaust. The pH value of saturated carbonic acid is pH56 which is why we define rain water with pH.
Normal rainwater has a ph value of between 5 6-acid rain has readings below 5. The term acid rain is used to describe all precipitations- rain snow fog dew- which are more acidic than normal water. Any acid rain with lower mathrmpH would be caused by additional acids.
Rain and snow the principal sources of ground water have pH values near 56 if they are relatively free of pollution. The most acidic rain falls in the eastern third of the United States with the region of lowest pH being roughly the states along the Ohio River valley. Acid rain has a negative connotation.
Thus it forms acid rain. The low pH of acid rain is due to sulfur oxides and nitrogen oxides and it is indeed below 57. When the pH of rain water drops from 5.
Damage their leaves limit nutrients expose to toxic substances like aluminum effects of acid rain on trees and forests. What is Acid Rain. It is slightly acidic because carbon dioxide CO 2 dissolves into it forming weak carbonic acid.
Rotting vegetation and erupting volcanoes release some chemicals that can cause acid rain but most acid rain is a product of human activities. Calculate the ratio of the hydronium ion in acid rain to that in normal rain. Acidity and alkalinity are measured using a pH scale for which 70 is neutral.
Causes of acid rain. When carbon dioxide dissolves in water it creates an acid called carbonic acid which is classified as a weak acid.
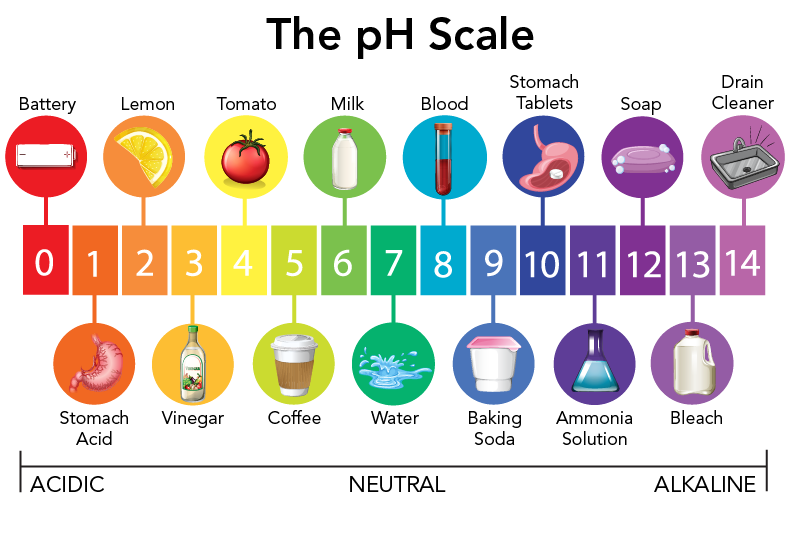
What Is Acid Rain Let S Talk Science

Ph Kennebec Estuary Land Trust

Topic 6 4 Acid Deposition Starter What Ph S Are Acidic Ppt Download

Contents Review Of Ph Definition Of Acid Rain Pollutants That Create Acid Rain A Sulfur Dioxide B Nitrogen Oxide C Ammonia Iv Acid Rain Ecosystem Ppt Download

What Is Ph Balance Why It S A Must While Washing Your Private Parts Spruce
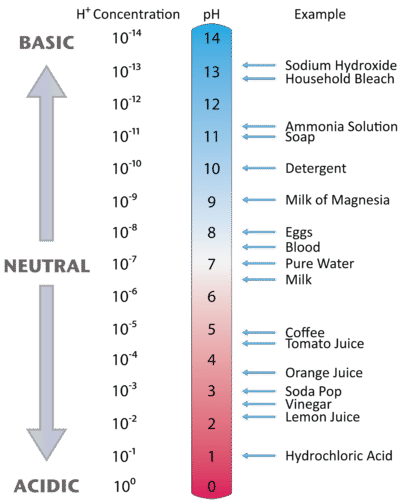
14 9 The Ph And Poh Scales Ways To Express Acidity And Basicity Chemistry Libretexts

Archived Environment And Climate Change Canada Air Acid Rain Faq
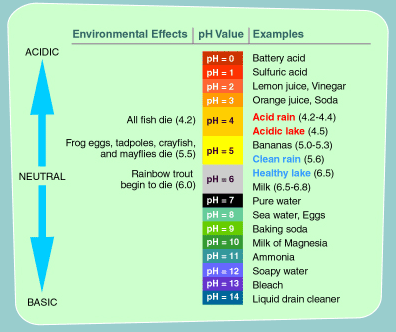
Acid Rain Students Site Ph Scale

6 6 But Acid Rain Can Have Ph Levels Lower Than 4 3 Where Is The Extra Acidity Coming From The Most Acidic Rain Falls In The Eastern Third Of The United Ppt Download
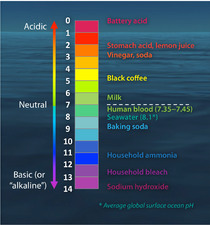






Comments
Post a Comment